Face
Skin
Hair
Hair Restoration
Women’s Eyebrows
PRP
Hair Restoration
Women’s Eyebrows
PRP

At Imago Medical, we use FDA-compliant regenerative products to help promote a healthy cellular environment that supports the body’s natural repair processes.
Stem Cell Therapy
Exosomes/Secretomes
Acoustic Shockwave Joint Treatment
NAD+
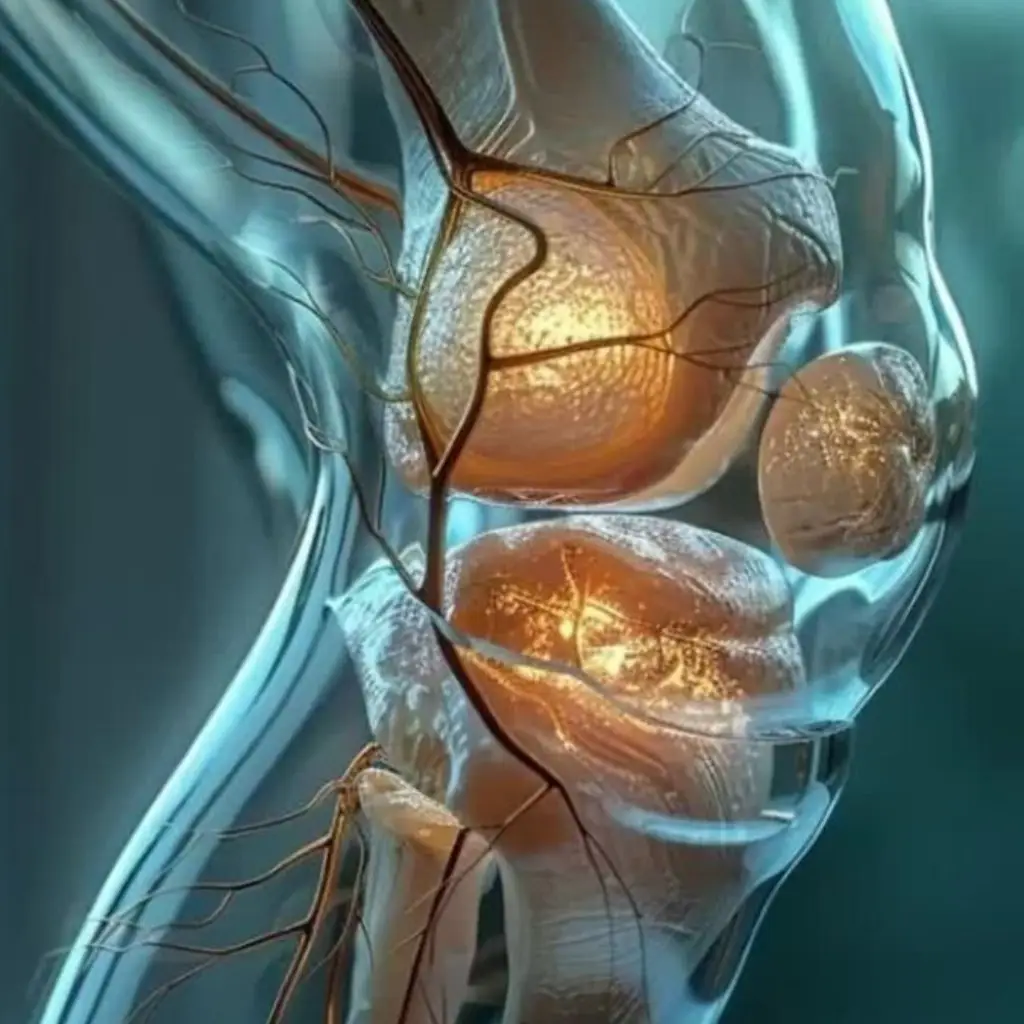
Imago’s Regenerative Medicine is the premiere stem cell therapy in Salt Lake City. We utilize Mesenchymal Stem Cell’s and their derivative signaling molecules that support your body’s natural regenerative & repair processes. Promoting cellular communication, tissue resilience, to support the body’s regenerative capability and assist a regenerative environment in joint tissue
Most importantly, we offer only original, non-cloned stem cells at the highest viability for best results. We do not add upsell treatments that waste your money without changing the outcome. Full transparency and superior quality.
Read on to learn the facts about stem cell therapy.
Multipotent MSC’s:
Multipotent mesenchymal stem cells (MSCs) are known for their ability to self-renew and differentiate into several cell types, including bone, cartilage, fat, and muscle.
Exosomes:
Exosomes are nanosized vesicles released by MSCs that contain biologically active proteins, lipids, and signaling molecules. They act as messengers — helping facilitate cellular tissue balance and recovery.
Our exosomes have been differentiated into tissue specific signals for greater stimulus: Muscle/Bone, Skin, Hair, Neural & Others
Secretomes:
The MSC secretome is the complete collection of bioactive substances secreted by these cells which collectively help support a regenerative and anti-inflammatory environment

| Metric (6–12 months) | Average Improvement | Source |
|---|---|---|
| Pain reduction (VAS 0–10) | 55–75 % | Matas et al., Stem Cells Translational Medicine 2025 (meta-analysis of 2,143 knees) |
| WOMAC function score | 45–68 % | Centeno et al., 2024 – 20M dose cohort |
| Patients with ≥50 % pain relief | 72–84 % | Utah Stem Cells internal audit 2023–2025 + Colorado clinic registry |
| Patients who avoid joint replacement for at least 2 years | 78 % | Davatchi et al., International Journal of Rheumatic Diseases 2024 |

Joint & Back Pain
Stem Cells & Joint Specific Exosomes
Acoustic Shockwave Therapy
Neuropathy
Stem Cells
Neural Specific Exosomes
I.V. Therapies
Regenerative Wellness
Blood Work
Stem Cells
Exosomes & Secretomes
NAD+ & I.V. Therapies
Sexual Wellness
Stem Cells & Tissue Specific Exosomes
Acoustic Shockwave
PRP/Botox
Safety First. Science Always.
We deliver the highest viable cells and full transparency on evidence based best practice. Our regenerative biologics are sourced and processed according to FDA guidelines for 361 HCT/P products. All treatments are administered by licensed medical professionals and designed to comply with FDA regulations.
Here is the truth about stem cell treatment, so you know what you are getting and why.

RELIEF THAT ACTUALLY REACHES THE AREAS
10-20 million live mesenchymal stem cells injected directly into and around your desired area — not filtered out in the lungs.
No cloning cells down. No I.V. wasted cells. No $4k+ upsells.
Why do we inject directly into the muscle/joint instead of I.V.?
80-95% of stem cells given intravenously are trapped in the lungs within minutes and never reach the desired areas.
• Study: Masterson et al. Mesenchylam Stroma Cells In Pulmonary Vasculature, 2021
• Study: Stanford PET imaging trial, 2024 → <3% o f IV-injected MSCs reach a knee at 24 hours
Direct intramuscular/peri-articular injection bypasses the lungs entirely, delivering 50-100x more live cells to the damaged tissue.
HOW DO WE KEEP THE STEM CELLS ALIVE & POTENT?
Cells are stored on-site at -80 °C in our cryofreezer.
We thaw only minutes before injection –
viability stays 92-97% (independent third-party testing).
Most clinics do a prolonged process or put them in I.V. fluids; viability drops 20-40% with prolonged time outside ultra-cold storage (Leibowitz et al., Cytotherapy 2024).
WHY DON’T WE ADD MRI, HYPERBARICS & OTHER ADDTIONS?
None of those have Level-1 evidence showing they improve outcomes when high-dose direct injection is already used.
We focus on what the data says actually works – getting
the maximum number of live cells to the joint – without expensive additions that don’t move the needle. We won’t waste your money. We deliver transparency & results.

Acoustic Shockwave Therapy is a cutting-edge, non-invasive treatment designed to stimulate your body’s natural healing processes. It also provides a much stronger signal to stem cell therapies to more fully go to the area, complimenting the treatment. Using targeted sound waves, this therapy helps:
At Imago Medical, we use targeted non-surgical therapy to treat stubborn joint pain, overuse injuries and chronic tendon issues – helping you recover faster, move better and possibly avoid surgery.
A next-generation solution for those ready to heal smarter.

At Imago Medical, we understand how joint pain, stiffness, and lingering injuries can hold you back — from the things you love and the life you want to live.
We combine the regenerative environment fostered by our MSC treatments with acoustic shockwave joint therapy to optimize your bodies renewal and recovery.
You don’t have to live limited. Let’s get you moving — better, faster, and stronger.
How Do Imago’s MSC’s Compare To I.V. Stem Cells Offered In Mexico & Panama?
Our stem cell therapy is preferred for the following reasons:
Our MSC+Exosome treatment is approximate signaling to high dose stem cells offered outside the U.S.
Are Stem Cells Safe?
Products outside of the U.S. not FDA regulated can carry much more risk. Trials of safety and effectiveness are ongoing. But for the U.S. based compliant products, a comprehensive systematic review of over 217 studies involving more than 4,500 human patients treated with MSCs (for various conditions) found no cases of tumor formation directly caused by injected MSCs, and no increase in tumor incidence compared to control groups. This was consistent regardless of MSC source (bone marrow, adipose, umbilical cord), delivery route (IV, intra-articular, etc.), or usage (autologous or allogeneic). This is initial evidence, more trials are needed. Here is the study link:
*This product is not FDA-approved to treat, cure, or prevent disease. Educational content only. Consult your licensed provider before treatment.
Results vary and are not guaranteed. These statements have not been evaluated by the Food and Drug Administration.